Scientific rheometer used to study the mechanical properties of biological materials in the micrometer range
Read moreRotational device that enables multidirectional tweezer measurements at high forces
Read moreTechnique to estimate the migration speed of different cell lines on 2D substrates
Read moreOne of the preconditions for a cell to survive and to grow is to attach and to spread on a substrate. Using their actomyosin machinery cells generate internal tension that contracts the cell body [1,2] and thus exert tractions on the underlying substrate through focal adhesions which physically connect the actin cytoskeleton to the ECM [3]. This traction force is crucial for cell shape maintenance, migration, mechanotransduction and many cellular functions. Traction force plays a important role in a lot of biological processes, such as inflammation, metastasis, wound healing, embryogenesis and angiogenesis. Therefore measuring the cell traction force is essential for understanding these underlying biological processes [4]. A very popular method to study these cell-substrate mechanical interactions, is with the use of polyacrylaminde hydrogels combined with fluorescent mirobeads as markers for determining the strain due to tractions forces [5] as originally described for use with silicone rubber sheets [6]. These gels are easy to employ [7,8] and have very good mechanical properties. Over a wide range of stress these gels produce a linear deformation and recover completely when stress is removed. By varying the concentration of bis-acrylamide the stiffness of the gel can easily be manipulated.
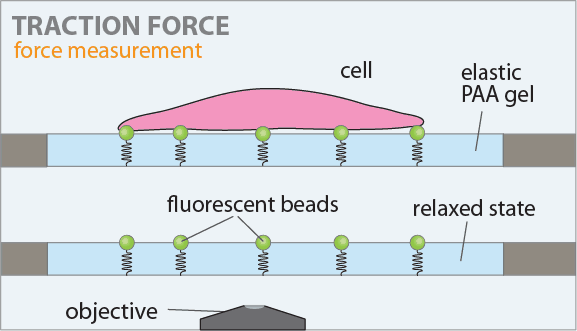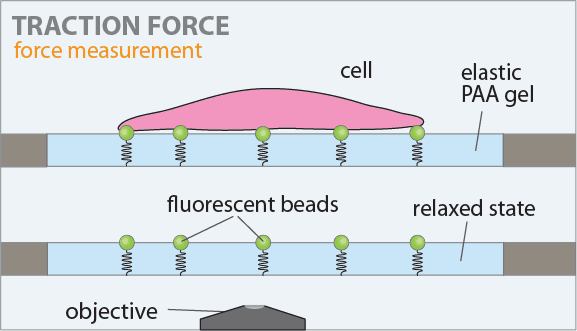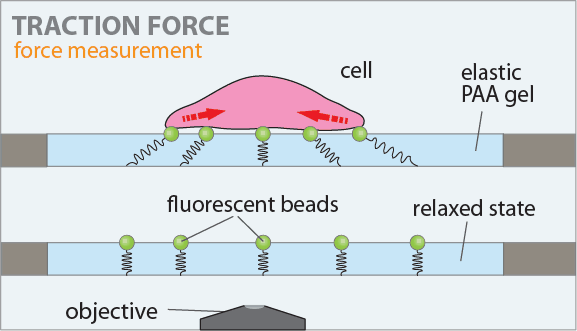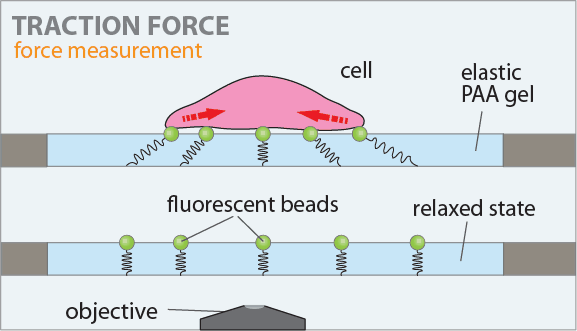
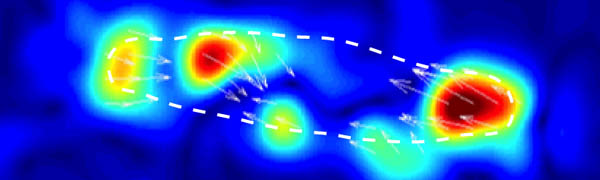
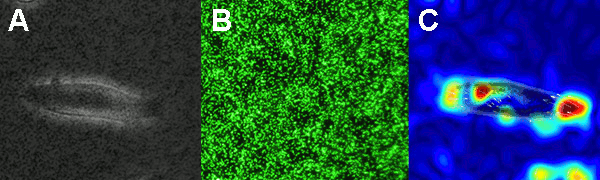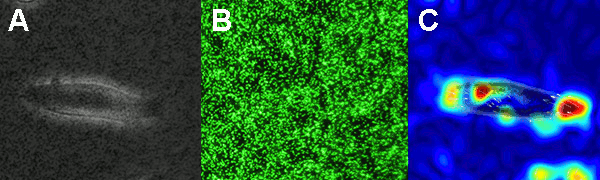
The figure on the left shows the principle of the traction force microscopy. In order to measure the contractile forces cells are seeded on an elasic PAA gel which is exactly determined in its mechanical properties and contains embedded beads on the surface. These beads serve as markers to visualize any deformation of the gel. When these cells start to spread they contract and thus deform the underlying substrate. Gel deformations are estimated using a Fourier-based difference-with-interpolation image analysis [9]. To characterize the contractile forces of each cell, the elastic strain energy stored in the polyacrylamide gel due to cell tractions is calculated as the product of local tractions and deformations, integrated over the spreading area of the cells [10].
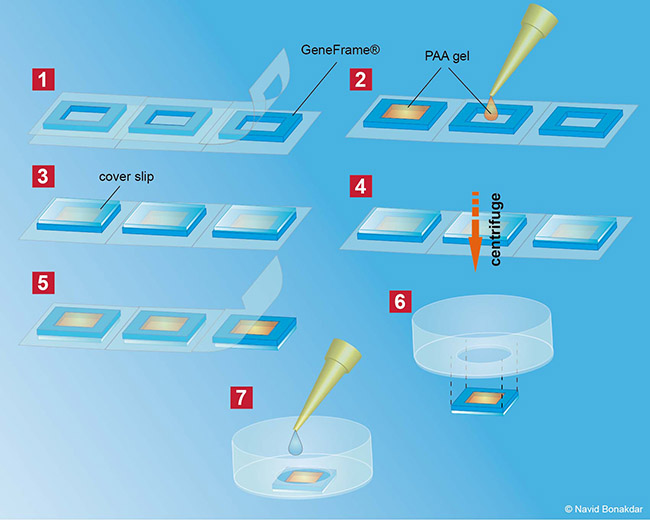
In our lab we produce our Poly-acrylamide gels directly in 35mm petri dishes. With this method many gels can be produced at once making measurement as well as storage very easy and steril. In order to use the PAA gels without a confocal microscop, all fluorescent beads in the gel have to be in one single focal plane. Therefor the gels have to be centrifuged during polymerisation process to bring all beads to the surface of the gel.
After the gels are fabricated, the surface of the gels is activated with sulfo-SANPAH (Pierce Biotechnology, Rockford, IL) and coated with with an extra cellular matrix protein e.g. fibronectin. Cells are then seeded on top of the coated polyacrylamide gels and are cultured for 1h-24h to let them completely adhere. Cell tractions are computed from an unconstrained deconvolution of the gel surface displacement field measured before and after cell detachment with 8 mM Cytochalasin D and Trypsin/EDTA (0.25/0.02%) in PBS [10]. During the measurements, the cells are maintained at 37°C in humidified atmosphere containing 5% CO2 .